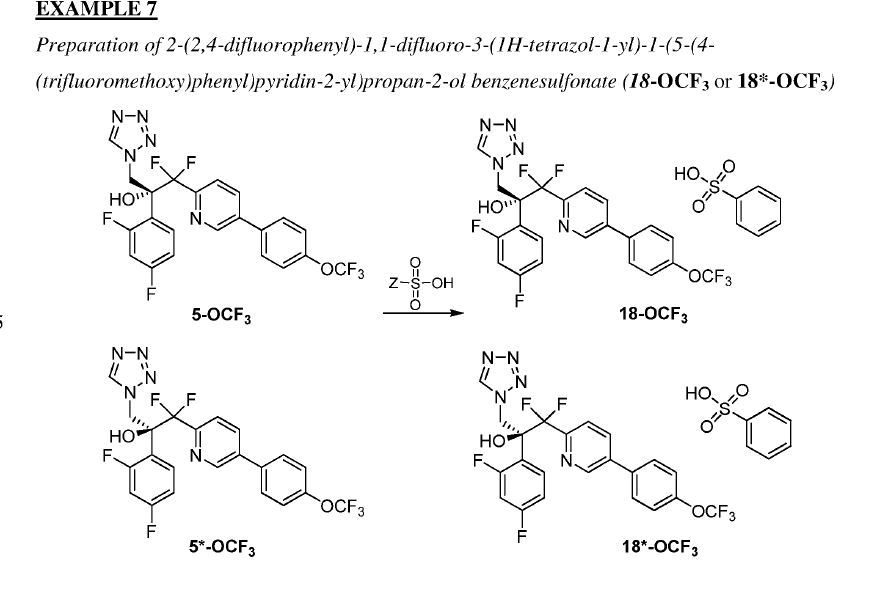
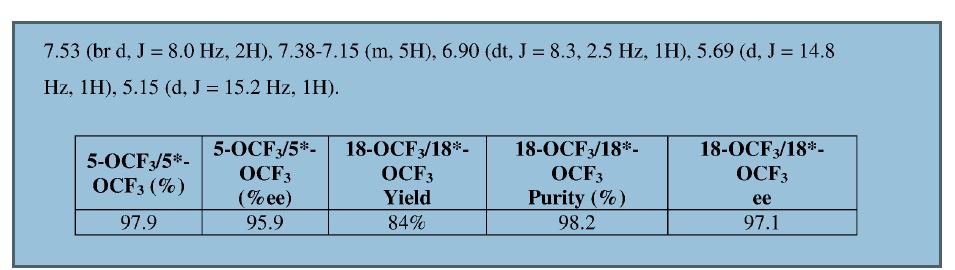
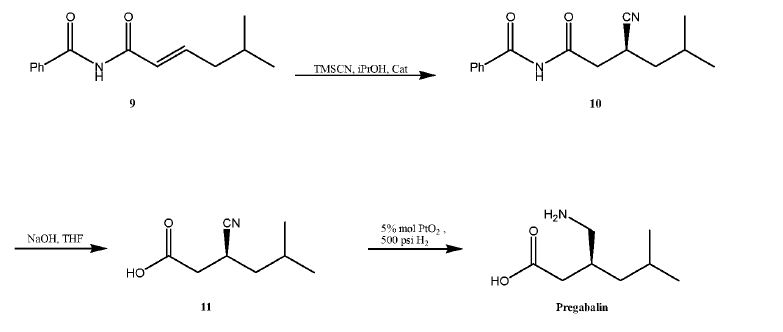
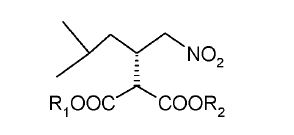
NEW PATENT, PONESIMOD, CRYSTAL PHARMATECH, WO 2017107972
Novel crystalline forms I, II and III of ponesimod . Useful as a selective sphingosine-1-phosphate receptor-1 (S1P1) receptor agonist, for the treatment of psoriasis. Appears to be first filing from Crystal Pharmatech claiming ponesimod. Johnson & Johnson , following its acquisition of Actelion , is developing ponesimod (phase III clinical trial), a S1P1 agonist, for the treatment of autoimmune disorders.
| Applicants: | CRYSTAL PHARMATECH CO., LTD. [CN/CN]; B4-101, Biobay, 218 Xinghu Street, Suzhou Industrial Park Suzhou, Jiangsu 215123 (CN) |
| Inventors: | CHEN, Minhua; (CN). ZHANG, Yanfeng; (CN). LI, Jiaoyang; (CN). ZHANG, Xiaoyu; (CN) |
Most of the family members of the product case ( WO2005054215 ) of ponesimod expire in European countries until November, 2023 and in the US by December, 2024 with US154 extension.
Disclosed are crystalline forms 1, 2, and 3 of a selective S1P1 receptor agonist, namely Ponesimod, and a method for preparing the same. An X-ray powder diffraction pattern of the crystalline form 1 has characteristic peaks at 2 theta values of 18.1° ± 0.2°, 14.6° ± 0.2°, and 11.3° ± 0.2°. An X-ray powder diffraction pattern of the crystalline form 2 has characteristic peaks at 2 theta values of 3.8° ± 0.2°, 10.8° ± 0.2°, and 6.1° ± 0.2°. An X-ray powder diffraction pattern of the crystalline form 3 has characteristic peaks at 2 theta values of 12.2° ± 0.2°, 6.2° ± 0.2°, and 5.6° ± 0.2°. Compared with existing crystalline forms, the present invention has better stability and a greatly increased solubility, and is more suitable for development of a pharmaceutical preparation containing Ponesimod
Ponesimod (compound of formula I) is a selective S1P1 receptor antagonist developed by Actelion. The drug was used to treat moderate to severe chronic plaque psoriasis in the two medium-term trial was successful, and will carry out the treatment of psoriasis in 3 clinical trials.
The present invention discloses a process for the preparation of a compound of formula I, which is disclosed in patent CN 102177144B, which is an amorphous form prepared by the process of CN100567275C, and discloses a process for the preparation of a compound of formula I, crystalline form C, crystalline form III, Type II. The results show that the crystallinity of crystalline form III is poor and it is converted to crystalline form II at room temperature. The crystalline form II is difficult to repeat and prepare a certain amount of propionic acid. The thermodynamics stability of crystalline form A is inferior to that of crystal form C. In contrast, For the crystal form suitable for the development of the drug, the solubility of the crystalline form C is not ideal.
Example 1
[0060]
Preparation of Ponesimod Form 1:
[0061]
48.1 mg of Ponesimod was added to 0.40 mL of 1,4-dioxane and the filtrate was filtered. To the solution was stirred at room temperature, 1.20 mL of n-heptane was added dropwise to precipitate the crystals and stirred overnight. The supernatant was filtered off by centrifugation Liquid to obtain Ponesimod crystal form 1.
Follow "'2014' Suzhou International Elite Entrepreneurship Week" with interest Over 88 billion venture capital investment helps your pioneering dreams come true
Since 2009, there have been 1267 overseas high-level talent projects settled in Suzhou through International Elite Entrepreneurship Week and 54 talents have been introduced and fostered for the national "Thousand Talents Plan". Among these 53 talents, Dr. Chen Minhua, the founder of Suzhou Crystal Pharmatech Co., Ltd., was deeply impressed by thoughtful services in Suzhou for innovative pioneering talents when he recalled the development in Suzhou. "Investment and financing services are placed with particular importance. Everything is thoroughly considered for fear that enterprise
In 2010, Chen Minhua quitted his job in a well-known pharmaceutical company in the United States and returned with his core 4-people R&D team. He founded Crystal Pharmatech Co., Ltd. in Suzhou Biobay through the Entrepreneurship Week. Till 2013, Crystal Pharmatech has made profits year by year. The yearly output value in 2013 reached 18 million Yuan, while the profits reached as high as 4 million Yuan. His clients involve half of top 20 pharmaceutical companies globally. Chen Minhua longs to fill the vacancy of drug crystals in China and take the lead in the international drug crystal research. Chen Minhua introduced that government service is an integral part to his growth. "Since it was settled down, Suzhou public sector organized several investment and financing activities and offered training and services in various aspects like the mode of financing, finance docking and enterprise strategic investment, which laid a solid foundation for Crystal Pharmatech's capital expansion", said by Chen Minhua.
To help high-level talents solve financial difficulty, Suzhou lays stress on the docking of science & technology and finance. The person in charge of the Municipal Science and Technology Bureau said that Suzhou guides and integrates social capital for equity investment of hi-tech enterprises at the start-up stage via the guiding funds set up by the government and follow-up investment, etc, thus evolving the venture capital investment cluster based on Shahu Equity Investment Center. After the national "Thousand Talents Plan" venture capital investment center was set up, pioneering talents and venture capital are further converging here. As of the end of 2013, there are 270 effective organizations engaged in various venture capital investment in Suzhou that manage the funds in excess of 88 billion Yuan. 30 million Yuan will be appropriated from the municipal science and technology fund budget for the newly established FOF of Angel Investment this year, so as to take avail of social capital for the development of small and medium-sized hi-tech enterprises.
Meanwhile, Suzhou sets up the special compensation fund against credit risks and offers "Kedaitong" with "low threshold and low interest rate", so as to solve financial difficulty of small and medium-sized hi-tech enterprises and create favorable financing environment for the pioneering work of talents and corporate development. At present, the fund of credit risk pool has reached 500 million Yuan and "Kedaitong" loans of 8.52 billion Yuan have been granted for 1023 small and medium-sized hi-tech enterprises. Particularly, 120 pioneering enterprises that feature independent intellectual property, high content of technology and light assets were backed up with 1.314 billion Yuan, the special risk compensation fund of "Kedaitong", thus vigorously supporting innovation and pioneering work of leading talents in the science and technology community in Suzhou.
Reporter Qian Yi
Quoted from Suzhou Daily on July 6, 2014
Quoted from Suzhou Daily on July 6, 2014
///////////